
Before labs buzzed with advanced organic synthesis, chemists relied on serendipity and incremental progress to find new compounds like 3,4,5-Trimethoxybenzoic acid methyl ester. Early records from the 1930s and 1940s tie the substance to natural product studies and side products from gallic acid transformations. Work done during the rapid expansion of organic chemistry led to its classification and explorations into its structural curiosity: three methoxy groups attached to a benzoic acid framework. Pharmaceutical and dye chemists charted much of this territory well before high-throughput tools made structure-property mapping routine.
This compound, better recognized as methyl mesitoate among some researchers, carves out a niche as both a chemical starting block and a research standard. With a backbone that’s been re-engineered in countless synthesis projects, it catches the eye for its reliability in providing functional groups amenable to a wide degree of downstream chemistry. Many reference labs keep it on hand, seeing value both as an intermediate for more complex pharmaceuticals and as a probe in method validation for HPLC and NMR analysis.
3,4,5-Trimethoxybenzoic acid methyl ester usually appears as a colorless, crystalline powder. Under the right conditions, its crystals stack neatly, offering decent purity without much purification drama. Melting points typically hover between 84°C and 88°C, confirming identity and serving as a crude check on sample integrity. The molecule weighs in at about 226 g/mol and, thanks to those three methoxy groups, resists excessive hydrogen bonding, remaining comparably soluble in hot ethanol, ethyl acetate, and chloroform. Chemically, its stability excels when stored dry and cool, sidestepping hydrolysis or decomposition that more fragile esters may face.
Commercial samples sport purity upwards of 98%, with meticulous HPLC and NMR checks governing release to market. Labels from credible suppliers don’t just list the CAS number and MFCD identifiers—they provide signal assignments, batch purity, and residual solvent content. For researchers accustomed to ambiguity in small-molecule procurement, these details help avoid redundant purification steps, shaving hours off their workflow. Recognized suppliers flag transport under ambient temperature since crystals withstand moderate shipping stress, but moisture-tight packaging never gets skipped.
Synthesizing this ester isn’t much of a stretch for most experienced benchworkers. The usual entry route uses 3,4,5-trimethoxybenzoic acid and an alcohol—most often methanol—under acid catalysis. Sulfuric acid or hydrochloric acid drive the conversion, and after a reflux period, base neutralization and water washing create a crude batch ready for crystallization. For those chasing extremely high purity, some opt for chromatographic purification, though most find recrystallization from ethanol sufficient. More elaborate approaches swap in alternative methylating agents, but these seldom improve yields or cost-competitiveness.
The strongly electron-donating methoxy groups not only calm the reactivity of the benzoic acid core, but also open unique doors for synthetic chemists. Electrophilic aromatic substitution, ordinarily a thicket of trouble, becomes more predictable. Bromination, nitration, or Friedel-Crafts acylation reactions progress with relative ease, and selective demethylation—from BBr3 or similar reagents—can tailor the aromatic ring environment. Hydrolysis under either acidic or basic conditions smoothly converts the methyl ester to the carboxylic acid, which expands its use across both academic and industrial contexts.
This ester often appears under a handful of names: methyl 3,4,5-trimethoxybenzoate, methyl trimethoxybenzoate, TMB methyl ester, and methyl mesitoate. Researchers sifting through supplier catalogs or journal archives need to keep an eye out for synonym drift—older papers and patents lean toward traditional IUPAC names or shorter trade names, so context draws from both systematic and commonplace nomenclature.
Few bench chemists line up excitedly to work with unknowns, and 3,4,5-Trimethoxybenzoic acid methyl ester checks boxes that foster confidence. Modern safety sheets identify a low acute toxicity profile and little in the way of environmental hazard so long as spills are controlled and waste policies followed. Gloves, goggles, and fume hood operation remain standard, as skin or eye contact with aromatic esters may still provoke mild irritation for some users. Good practice means not trusting luck when it comes to chemical handling, even with relatively tame substances like this ester.
I’ve seen this molecule circle through many a pharmaceutical research group, not just as a reference standard, but as a core for agrochemical intermediates and synthetic projects spanning material science to dye chemistry. In drug research, it shows up as a building block, making its way into anti-inflammatory and anti-tumor scaffolds. Probe development for chromatographic separation studies benefits from its defined retention and spectral properties. Some ecology labs use it as a calibration compound for environmental sample analysis, although its main footprint remains in organic chemistry and pharmaceutical development.
Continuous interest in new reaction conditions keeps research on this ester alive well past its "mainstream" routes. Catalysts seeking greener or more selective methylation pathways get benchmarked against the trusted acid-to-ester method. Medicinal chemists look for new derivatives, sometimes swapping out methoxy groups with bulkier or more reactive functionalities in search of novel biological properties. Analytical chemists lean on this compound’s reliability to fine-tune detection methods, particularly for GC-MS and LC-MS method development.
No researcher jumps into large-scale work without tracking down toxicity and environmental impact reports. To date, empirical data labels this ester as having low oral and dermal toxicity. Animal studies at high doses turn up only mild effects, with little evidence for cumulative or chronic organ damage. The molecule’s methoxy groups make metabolic oxidation more likely, so breakdown products—mostly harmless benzoic acid derivatives—help reinforce its reputation as a low-risk chemical. Nonetheless, responsible labs keep volumes down and engineer controls in place until new or long-term studies address all possible endpoints.
Brighter prospects for this ester seem anchored in two directions. First, green chemistry surges are spurring interest in developing more sustainable production processes—benign catalysts, alternative renewable starting materials, and energy-efficient purification schemes top the wish list. The other side rests with drug discovery: as aromatic cores get re-imagined for selective biological targeting, modifiers borrow liberally from the 3,4,5-pattern to tune properties or improve pharmacokinetics. Ongoing advances in computational modeling, combined with the ester’s stability and reactivity, promise expanded adoption in both standard and exploratory research. A molecule like this, with proven utility and adaptability, rarely goes out of style; it evolves with the needs and curiosity of whoever takes up the pipette next.
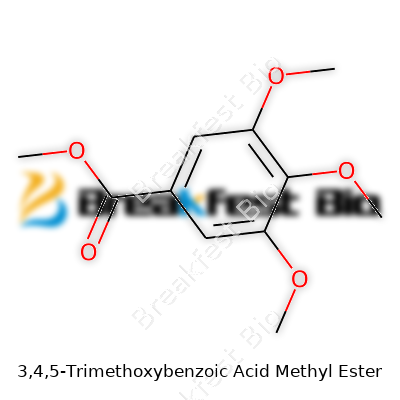
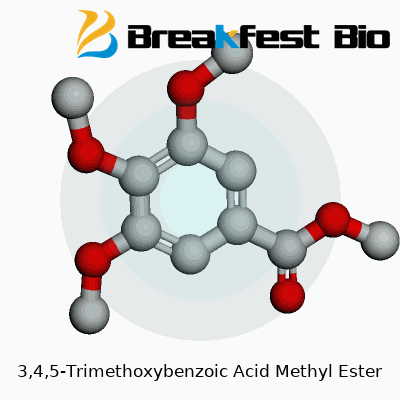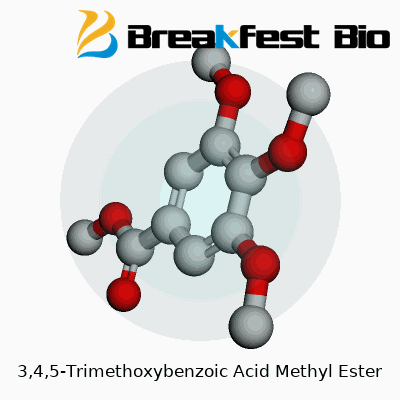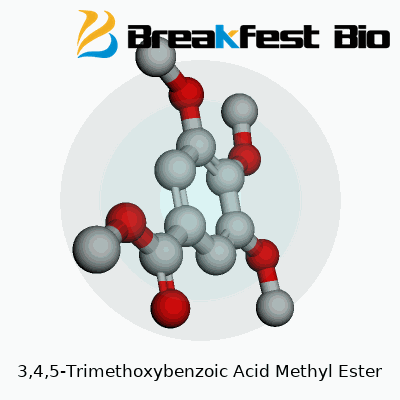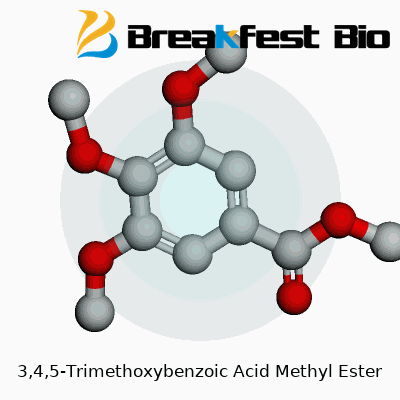
3,4,5-Trimethoxybenzoic acid methyl ester pops up in lab discussions and chemical catalogs, but its structure deserves a close look. The core of this molecule is a benzene ring—six carbons locked in a ring pattern, each with delocalized electrons. That stable, aromatic ring gives the compound its foundation. Attached to the ring, three methoxy groups cluster at positions 3, 4, and 5: these are –OCH3 branches adding electron-rich spots to the system. At one end, you find the methyl ester function instead of the standard carboxylic acid. This group—COOCH3—adds a distinct character, both chemically and physically.
The placement of those methoxy groups changes how this molecule behaves compared to plain benzoic acid. When I’ve worked with related compounds, these methoxy branches came in handy—they bump up solubility in organic solvents and tweak reactivity with other chemicals. The methyl ester, attached through an esterification step, seals the acidic carboxyl with a methyl group. As a result, the molecule loses some proton-donating power and gains resistance to basic hydrolysis. Once, a colleague synthesized this ester to test its stability in heated reactions—it performed far better than the parent acid under conditions that usually cause breakdown.
Laying this out on paper, the benzene ring carries –OCH3 groups on carbon 3, carbon 4, and carbon 5. The methyl ester group anchors at position 1. Chemically, that’s methyl 3,4,5-trimethoxybenzoate. The full molecular formula is C11H14O5. Visual models help clarify these attachments in three dimensions. Seeing how those methoxy arms splay out around the ring reveals why the molecule resists certain reactions. Stacking three oxygen-bearing groups close to each other changes electron flow across the ring, making the core less prone to substitution or oxidation in some settings.
Structural tweaks have real-world impact. Some pharmaceutical precursors use trimethoxybenzoate esters to anchor helpful side chains or mask problematic reactivity before further processing. In my own undergraduate synthesis, swapping a carboxylic acid for its methyl ester cut down on side reactions and boosted final yields. These transformations build on careful understanding of each chemical bond and functional group. Scientists across the world rely on detailed structures to predict outcomes and prevent costly mistakes in both industry and academia.
Students and lab techs sometimes stumble when visualizing organic structures. Access to online structure-drawing apps and 3D models can clear up these hurdles. Sharing richer visual resources and emphasizing the why behind each group’s role equips learners to troubleshoot, innovate, and communicate complex chemical stories more clearly. Whether for building new medicines or studying plant metabolites, fluency with structural diagrams leads to stronger results and fewer surprises.
Chemistry can seem distant, but some compounds earn their way into everyday relevance. Take 3,4,5-Trimethoxybenzoic acid methyl ester. Even if the name sounds like something from a lab textbook, it’s got real gravity within pharmaceutical research and chemical manufacturing. Chemists and process engineers don’t just keep it in the background. They turn to it for specific tasks because of its unique structure and reactivity.
This compound steps up as a building block for more advanced molecules. In drug development, it becomes a starting material for synthesizing analgesics and other biologically active compounds. For example, during the search for better anti-inflammatory drugs, researchers often rely on the trimethoxy pattern found in this molecule, as it closely resembles the molecular frameworks that occur in natural products like gallic acid derivatives or certain plant-based medicines. Those three methoxy groups support functionalization, offering spots for chemists to add or tweak chemical structures as they test for improved activity or lower toxicity. Journals covering medicinal chemistry mention this compound in connection to projects on new painkillers and anti-platelet agents. Some academic groups report using it to design molecules targeting the body’s central nervous system, underscoring its importance outside the factory setting.
Beyond medicine, the food and personal care industries have turned to 3,4,5-Trimethoxybenzoic acid methyl ester for synthesizing specialty chemicals. For example, some of the more subtle flavoring agents and aromatic compounds in lotions and perfumes start with esters like this one. Its mild odor and stability make it an attractive precursor for ingredients intended to deliver soft or woody notes. Years ago, people overlooked the chemistry behind fragrances, but with regulations tightening, companies look for reliable, well-characterized starting materials, and this ester fits the bill, in part because chemists know what to expect from its reactions.
Anyone who’s worked in a research chemistry lab has probably handled methyl esters for protecting carboxylic acids during multi-step syntheses. 3,4,5-Trimethoxybenzoic acid methyl ester does more than just sit on the shelf; it shields reactive groups while chemists build complex molecules. Later, under the right reaction conditions, the protecting group gets removed, leaving a clean product. This method helps chemists build intricate pharmaceuticals or test new reactions without the chaos of unwanted side reactions. Laboratory techs learn to appreciate these stable esters early, especially when yield and purity sit on the line.
Safety and environmental impact always matter in the modern world. Researchers track biodegradability and toxicity, since regulations have grown stricter for chemical manufacturing and export. Evidence up to now suggests this methyl ester doesn’t break down into particularly stubborn pollutants, but that’s not an invitation for carelessness. Manufacturers set up containment and recycling streams, especially when making precursors for drug candidates. Product stewardship departments keep an eye out for new findings, not only to safeguard workers but because consumers ask tough questions about what ends up in drug or fragrance supply chains.
Plenty of room for improvement remains. Green chemistry advocates push producers to rely more on renewable resources during synthesis. Labs test alternative solvents and reaction conditions. It’s now common for producers to measure carbon footprints and search for routes that shrink waste. Researchers keep an eye out for better ways to make or use this molecule, as both safety standards and demand keep shifting.
3,4,5-Trimethoxybenzoic acid methyl ester pops up pretty often in pharmaceutical research and organic chemistry labs. Its structure—three methoxy groups stuck to a benzene ring and a methylated carboxylic acid—gives it a unique fingerprint. Figuring out its molecular weight means adding up all those atoms: carbon, hydrogen, and oxygen. For this compound, the molecular formula is C11H14O5. Take each atom: 11 carbons at about 12.01, 14 hydrogens at roughly 1.008, five oxygens at 16.00. Add those values and you land at a molecular weight of about 226.23 g/mol.
That number isn’t just trivia. Chemists rely on this molecular weight in day-to-day work—prepping reagents, scaling reactions, calculating dosages. Accuracy here affects yield and quality down the line. In pharmaceutical work, a mistake in weight trickles forward; a slip early in synthesis can mean wasted time, failed tests, and a whole lot of frustration. It isn’t glamorous, but it’s the foundation for solid research.
My early days in the lab taught me just how unforgiving synthesis can be. A calculation error, maybe from copying a molecular weight off the wrong line, doesn’t seem like a big deal until you wonder why a reaction yields nothing or why analysis flags odd results. That experience made me a stickler for reviewing each step, starting from the molecular weight. It saves money and sanity.
The demand for accuracy increases at scale. Companies making pharmaceuticals or producing flavors and fragrances use 3,4,5-Trimethoxybenzoic acid methyl ester for its stability and reactivity. Every process uses the molecular weight as a starting point for batch calculations. Lax standards invite regulatory trouble and lost profit. According to the FDA, errors in quality control tied to miscalculation pop up more often than most folks realize. The numbers might feel routine, but the risks are concrete.
Being detail-oriented around molecular weights supports traceability. GMP regulations require clear, accurate documentation—from the purchase of starting materials to the shipment of the finished product. Digital tracking has helped, but software is only as good as the numbers entered. I remember an instance where a simple data entry mistake, a single digit off, brought production to a halt while we retraced steps across multiple batches. Fixing the process meant plugging these foundational errors from the start—in the calculation of molecular weights.
Cross-checking calculations should be second nature. In the lab, double verification is worth the effort. Calculator apps, open-access databases, and chemical inventory software minimize slips but don’t replace critical thinking. Encouraging young researchers to understand molecule structures lets them spot weird numbers before problems spread downstream. Training sessions on common calculation errors prove more valuable than some might expect—they stop mistakes before they snowball.
Bench chemists and production engineers alike benefit from a culture that values small details. For 3,4,5-Trimethoxybenzoic acid methyl ester, the number 226.23 g/mol reflects hard data and practical need. It isn’t about memorizing values but about knowing why they matter and how each step, no matter how simple, shapes the end result. A good molecule starts with a good calculation; everything else builds from there.
Anyone who’s worked with specialty chemicals like 3,4,5-Trimethoxybenzoic acid methyl ester knows how much real-world experience matters in a lab. Over the years, I've learned that sloppy storage wipes out even the best science. It only takes one mistake—a poorly sealed cap, a mislabeled bottle, a shelf too close to sun or heat—to turn a useful reagent into either an expensive waste or, worse, a hazard to everyone present.
Once, during a project in a university organic chemistry lab, someone left an aromatic ester on a bench in direct sunlight. That chemical didn’t just degrade; it let off an odor none of us could ignore. Now, 3,4,5-Trimethoxybenzoic acid methyl ester is usually more stable than some reactive compounds, but proper storage keeps both its physical integrity and your team’s safety intact.
This compound tends to hold up well at room temperature, but fluctuations and warmth speed up breakdown. A cool, dry cabinet beats an office shelf every time. Laboratories set their storage between 2-8°C—standard for sensitive reagents. Direct sunlight breaks apart aromatic compounds faster than people expect, so I always choose an opaque or amber container. It’s a simple fix that pays off: less degradation, longer shelf life, clearer results, and less money down the drain.
Humidity causes subtle issues, especially for powders and crystalline esters. If water gets into the vial, hydrolysis can occur even with an ester as robust as this one. Adding a desiccant packet helps, but it's not a substitute for a tight cap and thoughtful placement. I learned to double-check storage instructions every time a new reagent shows up. Just because the bottle looked clean when shipped doesn't mean it's safe forever on a shelf. For many chemicals, that extra step prevents headaches down the line.
Mislabeled reagents waste more time than almost anything else. A clear label—name, concentration, arrival date, and owner—turns a risky unknown back into a useful lab tool. Some labs use barcode systems, others stick with permanent markers. Either way, knowing exactly what’s in each jar means mistakes don’t snowball.
People rarely talk about it, but labels and records deliver the trust that research relies on. During busy seasons, I've seen new team members tempted to skip details. Every single time, that shortcut led to mixups, angry emails, and panicked scrambles for substitutes. Transparent documentation and ownership becomes routine after the first close call. Simple as it sounds, a sharpie and a routine check cut down on everything from accidental misuse to potential safety violations.
Everyone spills something eventually, no matter how careful. Early in my career, I wiped up a spill with a paper towel and thought nothing of it. Later, I noticed a headache and an odd taste in the air. Proper procedure calls for fume hoods, gloves, and disposal according to guidelines, especially with aromatic esters. 3,4,5-Trimethoxybenzoic acid methyl ester isn’t extreme on the hazard scale, but that never justifies shortcuts.
No one can remember every detail, so shared digital logs and weekly lab walkthroughs backstop individual oversight. Safety, longevity, and research quality all spike when storage becomes a habit, not an afterthought. Taking ten minutes for these checks pays for itself many times over—less waste, fewer accidents, and science everyone can trust.
We hear plenty about chemical safety, especially when it comes to organic compounds that land somewhere in research labs or specialty industries. One common question pokes at 3,4,5-trimethoxybenzoic acid methyl ester, a mouthful of a substance often used as an intermediate in lab syntheses or certain fine chemical applications. People worry whether compounds like this actually pose a threat to health or safety, and information online doesn’t always clear things up.
Scrolling through technical safety sheets and chemical supplier data, it's easy to get overwhelmed by hazard symbols and safety jargon. With this compound, evidence offers a mixed message. Many Material Safety Data Sheets (MSDS) list its hazard as being relatively mild—possibly causing irritation if inhaled, swallowed, or if it lands on skin or in the eyes. So it’s not exactly a poison to handle with tongs, full-face respirators, and prayers. Still, nobody enjoys unprotected contact with random chemicals, even those labelled “low hazard”.
Looking at toxicity numbers, 3,4,5-trimethoxybenzoic acid methyl ester doesn’t throw up red flags. According to laboratory studies, the substance lacks strong acute toxicity; the available animal tests show relatively high LD50 values, which means it takes a pretty hefty dose to cause serious harm. In routine research settings, exposure happens only rarely and typically at low amounts—much less than would cause severe problems. For perspective, plenty of household cleaning products pack a bigger punch in toxicity.
Even if a chemical gets categorized as “low risk”, habits in handling count for a lot. Small spills, accidental inhalation, or eye contact all present minor but annoying risks like irritation or temporary discomfort. That makes gloves and goggles more than just annoying formalities. In my own time working with organics, I slipped up once and caught a mild chemical splash—nothing dramatic, just a sore hand for a few hours. It taught me that being careful isn't about fear; it’s about avoiding unnecessary discomfort or distraction.
When chemicals are handled in educational settings, such as undergraduate labs, safeguards often include fume hoods and clear labels. No one expects these compounds to send someone to the hospital from a splash, but encouraged habits mean fewer headaches and, in rare scenarios, fewer trips to see the campus nurse. Besides, good practices learned now pay off with nastier materials later in a career, or in an industrial setting.
The truth is that much of the worry around chemicals relates to how they’re labeled and perceived. Regulatory bodies like the European Chemicals Agency track substances for known dangers. If 3,4,5-trimethoxybenzoic acid methyl ester showed strong evidence of lasting harm, those warnings would show up loud and clear at the register. Studies so far just don’t raise alarms. It isn’t persistent in the environment and breaks down without leaving much behind. Authorities pay more attention to solvents, heavy metals, and known carcinogens.
Keeping up with safety doesn’t only mean reading labels. Sharing updated MSDS documents and encouraging a culture of using protection gear cuts down on both short- and long-term risks. Mistakes happen most often when people feel too confident in their knowledge and skip over routine checks. Relying on training and clear communication helps prevent that confidence from turning into trouble.
Addressing the actual risk means more than a blanket warning or hyped caution. Keep the containers sealed, respect local rules in disposal, avoid exposure to skin and eyes, and work under fume hoods if dust or fumes have a chance to show up. Keep protective gear within arm’s reach, not locked away in a cabinet. In labs where new students or staff rotate in, regular review of the basics can prevent more problems than trying to memorize every potential hazard.
For most people outside chemistry labs, contact with 3,4,5-trimethoxybenzoic acid methyl ester never comes up—and that’s as it should be. For those who do work around it, treating even “low hazard” compounds with simple respect keeps discomfort and accidents to a minimum.
| Names | |
| Preferred IUPAC name | Methyl 3,4,5-trimethoxybenzoate |
| Other names |
Methyl 3,4,5-trimethoxybenzoate
Methyl Eudesmate Methylmescalate |
| Pronunciation | /ˌtraɪ.mɛθ.ˈɒk.si.bɛnˈzoʊ.ɪk ˈæs.ɪd ˈmiː.θəl ˈɛs.tər/ |
| Identifiers | |
| CAS Number | 1916-07-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **3,4,5-Trimethoxybenzoic acid methyl ester** (also known as **methyl 3,4,5-trimethoxybenzoate**) in **SMILES** notation: ``` COC(=O)C1=CC(=C(C(=C1)OC)OC)OC ``` |
| Beilstein Reference | 1256815 |
| ChEBI | CHEBI:38336 |
| ChEMBL | CHEMBL404350 |
| ChemSpider | 12645 |
| DrugBank | DB03796 |
| ECHA InfoCard | 03e627c8-b1ef-428e-abc1-2b5cffe5a1e7 |
| EC Number | 3.1.1.6 |
| Gmelin Reference | 77856 |
| KEGG | C07678 |
| MeSH | D014020 |
| PubChem CID | 7118 |
| RTECS number | DG9410000 |
| UNII | J6W7C3S9B5 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID9056981 |
| Properties | |
| Chemical formula | C11H14O5 |
| Molar mass | 226.23 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.22 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 2.15 |
| Vapor pressure | 0.0000178 mmHg (25°C) |
| Acidity (pKa) | pKa ≈ 4.2 |
| Basicity (pKb) | 14.42 |
| Magnetic susceptibility (χ) | -77.1 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5460 |
| Viscosity | 400 cP (20°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 285.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −549.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2132.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | Flash point: >110°C |
| LD50 (median dose) | LD50 (median dose): Rat oral 2120 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Gallic acid
Methyl gallate 3,4,5-Trimethoxybenzaldehyde 3,4,5-Trimethoxybenzoic acid 3,4,5-Trimethoxyphenol |